Investigating Cellular Response to Impact With a Microfluidic MEMS Device
Luke Patterson, Jennifer Walker, Evelyn Rodriguez-Mesa, Kevin Shields, John Foster, Megan T. Valentine, Adele Doyle, and Kimberly Foster. Journal of Micrelectromechanical Systems 29 14-24 (2020).
Abstract
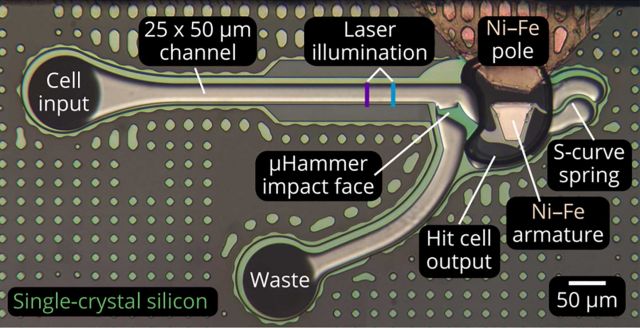
Although high strain and strain-rate impacts to the human body have been the subject of substantial research at both the systemic and tissue levels, little is known about the celllevel ramifications of such assaults. This is largely due to the lack of high throughput, dynamic compression devices capable of simulating such traumatic loading conditions on individual cells. To fill this gap, we developed and characterized a high speed, high actuation force, magnetically driven MEMS chip to apply stress to biological cells at unprecedented strain (10% to 90%), strain rate (30,000 to 200,000 s-1), and throughput (12,000 cells/min). To demonstrate the capabilities of the μHammer, we applied biologically relevant strains and strain rates to human leukemic K562 cells and then monitored their viability for up to 8 days. We observed significantly repressed proliferation of the hit cells compared to both unperturbed and sham-hit control cells, accompanied by minimal cell death. This indicates success in applying cellular damage without compromising the overall viability of the population, allowing us to conclude that this device is well suited to study the subtle effects of impact on large populations of inherently heterogeneous cells.